ALOPECIA HAIR LOSS TREATMENT IN TORONTO GTA
INTRODUCTION
Hair loss is a common concern affecting individuals worldwide, including the Toronto GTA region. Fortunately, there are various innovative treatments available to address this issue. PRP in hair treatment and AAPE® stem cell therapy are two special treatments gaining popularity. Using a non-surgical approach, PRP therapy involves injecting concentrated platelets and growth factors from the patient’s blood into the scalp to activate hair follicles and facilitate vibrant hair growth. On the other hand, AAPE® stem cell therapy utilizes growth factors extracted from human adipose-derived stem cells conditioned media to regenerate hair follicles and promote prompt restoration. Both treatments offer safe and effective alternatives for individuals experiencing hair loss.
Other options, such as the Pharma Hermetic Hair Recovery Program® and Nourkrin® Woman, also provide comprehensive solutions to strengthen hair follicles, improve hair health, and combat hair loss. Moreover, Theradome® laser hair therapy and SMP scalp micropigmentation offer additional possibilities for non-surgical hair restoration. This article explores these treatments in more detail and highlights their benefits for individuals seeking to address hair loss in the Toronto GTA region.
PRP IN HAIR TREATMENT
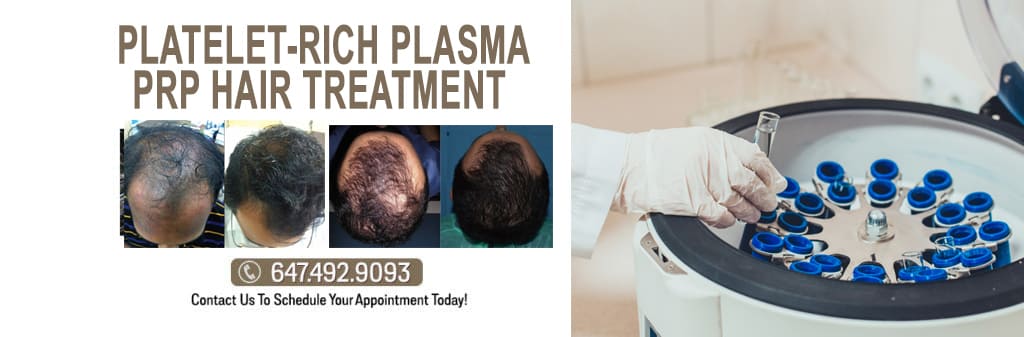
PRP therapy has become increasingly popular as a non-surgical treatment for early-stage hair loss or thinning. It provides a safe and natural alternative to hair transplantation. The process begins by collecting the patient’s blood and subsequently undergoing centrifugation to segregate the plasma from the red and white blood cells. The resulting solution, containing concentrated platelets and growth factors, is injected into the scalp at the hair loss or thinning site. This injection stimulates the hair follicles, promoting healthy new hair growth. The combined action of platelets and growth factors in PRP therapy invigorate hair follicles and supports the development of strong and healthy hair.
PRP therapy is a minimally invasive treatment requiring multiple sessions for optimal results. The number of sessions needed is determined by the severity of hair loss and the patient’s response to treatment. PRP therapy can also be combined with other hair restoration treatments, such as laser therapy, medication, or hair transplant surgery.
The advantages of PRP therapy for hair loss include its non-surgical nature, minimal recovery time, and a natural and safe treatment option.
PRP therapy has demonstrated efficacy in both genders and is particularly advantageous for individuals with female pattern hair loss or androgenetic alopecia. PRP therapy can also enhance the thickness and quality of existing hair, resulting in a fuller, healthier-looking head of hair for the patient.
What is the process of getting PRP therapy for hair loss?
The process of getting PRP therapy for hair loss typically involves the following steps:
- Consultation: Patients meet with a medical professional to discuss their hair loss and determine if PRP therapy is a suitable treatment option.
- Blood Draw: After drawing the patient’s blood, it undergoes a process of centrifugation, separating the plasma from the red and white blood cells.
- Injection: The PRP solution is injected directly into the scalp at the hair loss or thinning site.
- Follow-up: Patients may require multiple PRP therapy sessions over several months, with follow-up appointments to monitor progress.
The entire PRP therapy process usually takes 45-90 minutes and is performed outpatient. Patients can generally resume normal activities immediately following the procedure.
MORE INFO ON PRP
What is PRP therapy for hair loss?
PRP therapy for hair loss is a non-surgical treatment that involves injecting concentrated platelets and growth factors from the patient’s blood into the scalp. This stimulates hair follicles, promoting new hair growth.
How does PRP therapy work?
PRP therapy utilizes the growth factors and platelets in the patient’s blood. When injected into the scalp, these substances stimulate hair follicles, promoting healthier hair growth and reducing hair loss.
How many sessions of PRP therapy are needed for optimal results?
The number of PRP therapy sessions necessary differs based on the severity of hair loss and how each person’s body reacts to the treatment. Typically, multiple sessions are needed over a few months to achieve optimal results.
Can PRP therapy be combined with other hair restoration treatments?
Yes, PRP therapy can be combined with other hair restoration treatments such as laser therapy, medication, or hair transplant surgery. Combining treatments may provide enhanced results for certain individuals.
Is PRP therapy safe?
PRP therapy is considered safe as it uses the patient’s blood, reducing the risk of allergic reactions or other adverse effects. It is a low-risk procedure with minimal downtime. However, as with any medical treatment, there may be potential risks and side effects, which should be discussed with a healthcare professional.
AAPE® STEM CELL THERAPY
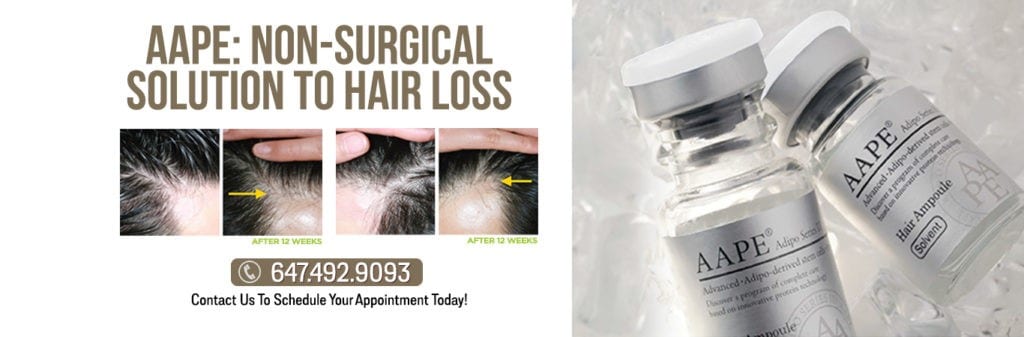
AAPE® is a stem cell technology that promotes hair follicles regeneration and prompt restoration. AAPE® is a mixture of refined growth factors extracted from human adipose-derived stem cells conditioned media. It induces the proliferation of dermal papilla cells of human hair follicles to increase total hair count and make hair regrow twice faster. AAPE® has:
- US FDA, CFDA Approval
- PCPC US registration
- 15 International SCI journals
- 28 Patents registration worldwide
The use of AAPE®, “fat” or adipose-derived stem cells conditioned media has been used successfully in treating female hair loss. The non-invasive treatment is described in the following journal article, including a new study and scientific analysis of hair growth measurements and results photos published in the International Journal of Dermatology. This advanced stem cell-derived protein extract called AAPE can now be applied in a clinical setting without discomfort or downtime as a lunch-hour session at the Toronto and Richmond Hill Trichology Centre | Hair Loss Clinic.
MORE INFO ON AAPE STEM CELL THERAPY
WHAT IS AAPE STEM CELL THERAPY, AND IS IT SAFE?
AAPE stem cell therapy is a safe and minimally invasive treatment that utilizes growth factors and cytokines extracted from human adipose-derived stem cells conditioned media. These naturally occurring components pose little risk of allergic reaction or adverse effects. AAPE stem cell therapy does not involve harsh chemicals or surgery, making it a safe alternative for individuals seeking hair restoration.
HOW EFFECTIVE IS AAPE STEM CELL THERAPY FOR HAIR LOSS?
AAPE stem cell therapy has shown promising results in treating hair loss. This therapy promotes the growth of new, healthy hair by stimulating hair follicles, resulting in improved hair density, thickness, and reduced hair loss. The administration of growth factors and cytokines in AAPE stem cell therapy increases blood flow to the hair follicles, leading to enhanced cellular activity and hair growth.
WHERE CAN I FIND MORE INFORMATION ON AAPE STEM CELL THERAPY?
For further details on AAPE stem cell therapy and its benefits, it is recommended to consult with a qualified healthcare professional or visit reputable sources that provide comprehensive information on the topic.
PHARMA HERMETIC HAIR RECOVERY PROGRAM®

Pharma Hermetic Hair Recovery Program® works at revitalizing the hair bulb and obtaining greater thickness and strength. It helps strengthen the anchorage of the root and rebalance the scalp.
In addition, the Hair Recovery Program® SP55 enhances the growth of new hair and activates dormant follicles thanks to its effective active ingredients. Providing all the necessary nutrients to repair weakened and brittle hair. After the treatment, the hair becomes thicker and more voluminous.
We rely on ingredients suitable to penetrate the scalp and stop hair loss.
- Thicker hair.
- More density.
- No side effects.
- Topical application.
- Lasting results.
MORE INFO ON PHARMA HERMETIC
WHAT IS THE PHARMA HERMETIC HAIR RECOVERY PROGRAM?
The Pharma Hermetic Hair Recovery Program is a comprehensive hair treatment program designed to rejuvenate the hair bulb, strengthen hair follicles, and rebalance the scalp. The collection of products included in the program delivers essential nutrients to strengthen and restore fragile hair. By addressing the root causes of hair loss, the program aims to achieve thicker and more voluminous hair.
HOW DO PHARMA HERMETIC PRODUCTS DIFFER FROM OTHER HAIR LOSS PRODUCTS?
Pharma Hermetic sets itself apart from other hair loss products by offering a distinct strategy for treating hair loss. Pharma Hermetic takes a different approach, unlike many products on the market that rely on strong chemicals or medications. Their products are specially formulated to target the root causes of hair loss, such as inflammation, nutritional deficiencies, and hormonal imbalances. Instead of harsh chemicals, Pharma Hermetic utilizes natural ingredients that stimulate healthy hair growth and promote scalp health.
NOURKRIN® WOMAN, FOR FEMALE HAIR LOSS

If you’re worried about certain hair loss medications that contain ingredients that may cause some side affects, such as Minoxidil, we offer a hair loss treatment called Nourkrin®. Nourkrin® contains natural ingredients and has been clinically proven to help thin hair. The key active ingredients are marine-based extracts of proteins and polysaccharides combined with silica, vitamin C, and horsetail extract.
Developed by scientists in Finland, Nourkrin® has been the subject of many double-blind, placebo-controlled clinical studies, the latest of which showed that 77% of all participants reported a positive effect during a six-month treatment period, and their hair count increased by an average of 35.7%. Nourkrin® Woman with the unique Marilex® helps support, normalize and maintain the Hair Growth Cycle by carefully providing the correct nutrients to the hair follicles. Nourkrin® is the only product worldwide containing Marilex® (which has a unique and protected composition structure), and consumers should therefore beware of imitation products that claim to deliver similar effects with so-called ‘marine proteins’ or ‘marine extracts’ – as these are not the original, scientifically tested Marilex®. Today, the award-winning Nourkrin® Woman is appreciated by women around the world. Nourkrin® Woman is completely safe and 100% drug-free. You can get the Nourkrin® Woman hair loss treatment at the Toronto and Richmond Hill Trichology Centre | Hair Loss Clinic.
What are the benefits of using Nourkrin Women?
Nourkrin Women is a nutritional supplement that promotes hair growth and prevents hair loss in women. Among the advantages of using Nourkrin Women are:
- Nourkrin Women contains a proprietary combination of marine extracts that have been shown to promote healthy hair growth.
- Strengthens hair follicles and improves hair’s overall health, resulting in denser, fuller hair.
- It can aid in reducing hair shedding, a prevalent issue for women experiencing hair loss.
- It is a natural and safe dietary supplement that contains no harsh chemicals or medications.
- Appropriate for all hair types: Suitable for all hair varieties and all ages of women.
- Nourkrin Women has been proven effective in clinical trials for promoting hair growth and reducing hair loss.
Overall, Nourkrin Women is a safe and effective option for women with hair loss or thinning. Its exclusive combination of marine extracts strengthens hair follicles, promotes healthy hair growth, and reduces hair loss, resulting in denser, fuller-looking hair.
MORE INFO ON NOURKRIN
What is Nourkrin for female hair loss?
Nourkrin is a hair loss treatment specifically designed for women. It is a nutritional supplement that promotes hair growth and helps prevent hair loss. Nourkrin contains marine-based extracts of proteins and polysaccharides, along with other beneficial ingredients like silica, vitamin C, and horsetail extract.
How does Nourkrin work?
Nourkrin supports, normalizes, and maintains the hair growth cycle by providing the necessary nutrients to the hair follicles. The efficacy of Nourkrin lies in its marine extracts, which have been proven to promote healthy hair growth, fortify hair follicles, and decrease hair shedding, resulting in hair that appears denser and fuller.
Is Nourkrin safe to use?
Nourkrin is a natural and safe dietary supplement with no harsh chemicals or medications. It has been clinically proven and extensively studied, demonstrating its effectiveness and safety in promoting hair growth and reducing hair loss.
THERADOME® LASER HAIR THERAPY
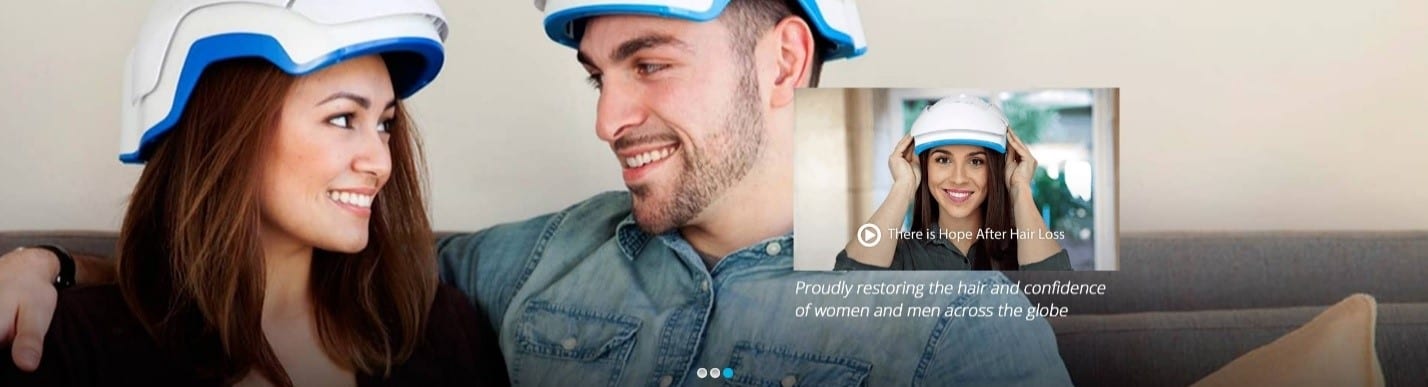
The Theradome® brings you the world’s most advanced laser hair growth treatment from the comfort of your home. Unlike Light Emitting Diode (LED) devices, our laser light targets the stem cells at the base of hair follicles. This allows the formation of a new photonic pathway that can restore hair to a healthy state. Theradome® one-of-a-kind laser hair helmet was engineered based on four crucial scientific criteria, providing the most powerful and efficacious laser hair growth treatment.
Only lasers produce coherent light, meaning their waves are aligned and travelling in synchronicity. Laser light is needed to penetrate the scalp at a depth of 3 – 5 mm and reach the base of a hair follicle. No other light source (i.e., LEDs) can achieve this. Our proprietary cold laser hair growth treatment ensures the mitochondria of hair cells are stimulated by treating the base of hair follicles. The mitochondria then produce cell energy that is harnessed by hair follicles. You can purchase the Theradome® hair loss laser therapy device at the Toronto and Richmond Hill Trichology Centre | Hair Loss Clinic.
MORE INFO ON LASER THERAPY
CAN THERADOME LASER HAIR THERAPY EFFECTIVELY TREAT HAIR LOSS?
Theradome laser hair therapy, utilizing low-level laser treatment (LLLT), has proven effective in addressing hair loss. In clinical trials, Theradome laser helmets have demonstrated the ability to stimulate hair growth by increasing scalp blood flow, promoting cellular activity, and enhancing hair development. These FDA-approved helmets have improved hair density, thickness, and overall health. It is important to note that the effectiveness of Theradome laser hair therapy may vary depending on the severity and underlying cause of hair loss. For optimal results, it is recommended to incorporate a balanced diet, regular exercise, and other medically prescribed hair loss therapies alongside Theradome treatment. Additional information on laser therapy can be found to understand this treatment option further.
SMP SCALP MICROPIGMENTATION
Scarring alopecia can be a complex condition for patients, as traditional hair restoration treatments may not be effective. However, an option is available for those seeking a non-surgical solution. SMP scalp micropigmentation is a cosmetic procedure that mimics the appearance of hair follicles by applying pigment to the scalp.
The technique involves depositing pigment into the dermal layer of the scalp, creating tiny dots that resemble hair follicles. This technique is most effective for women with dark brown or black hair and can add thickness to areas of thinning hair. SMP can also be an excellent choice for both men and women who want to reduce the appearance of areas of the scalp where more skin is showing.
While SMP is a safe and effective treatment, it is essential to note that the procedure is highly specialized and requires the expertise of a skilled professional. Applying the pigment requires high precision and artistry to achieve natural-looking results. Therefore, choosing a reputable and experienced practitioner to perform the procedure is crucial. In addition, the development of the treatment can significantly impact a patient’s self-esteem and confidence, making it imperative to choose the right provider.
Does scalp micropigmentation look real?
Yes, scalp micropigmentation can look real to an experienced and skilled professional. For this method, pigments are put into the head to make them look like hair follicles. Then, looking at the pigmented dots from an average distance, they can look like real hair shafts, giving the impression of a fuller head of hair.
One benefit of SMP is that it can be changed to match the natural colour and thickness of a person’s hair, making it look more realistic. Skilled professionals can also use various methods to make a hairline look more lifelike and natural.
It is essential to remember that achieving the most realistic outcome with SMP can depend highly on several factors, including the practitioner’s expertise, the quality of the pigments employed, and the individual characteristics of the patient’s scalp. As a result, conducting thorough research and selecting a practitioner with a solid reputation and extensive experience is crucial in obtaining the best possible outcome.
MORE INFO ON SCALP MICROPIGMENTATION
Is scalp micropigmentation a long-lasting solution for scarring alopecia?
Scalp micropigmentation can provide a long-lasting solution for scarring alopecia. By depositing pigment into the scalp to mimic the appearance of hair follicles, SMP can effectively camouflage the scars caused by scarring alopecia. The pigmented dots create the illusion of hair, giving the scalp a fuller and more natural look. However, it’s important to note that the longevity of SMP can vary depending on factors such as the individual’s skin type, sun exposure, and aftercare practices. It is important to schedule regular touch-ups to maintain the desired results.
Can scalp micropigmentation be customized to match hair colours and styles?
Scalp micropigmentation can be customized to match various hair colours and styles. Skilled professionals in SMP can adjust the pigment colour to closely resemble the individual’s natural hair colour, whether dark brown, black, or other shades. Additionally, they can use shading techniques and different needle sizes to create a realistic replication of hair follicles, allowing for a more natural and lifelike appearance. SMP aims to provide a personalized solution that blends seamlessly with the individual’s existing hair and complements their desired style.



CONCLUSION
The availability of advanced hair loss treatments in the Toronto GTA region provides hope for individuals struggling with hair loss. PRP therapy and AAPE® stem cell therapy offer non-surgical approaches that stimulate hair follicles and promote healthy growth. The Pharma Hermetic Hair Recovery Program® and Nourkrin® Woman provide comprehensive solutions to strengthen hair follicles, improve hair health, and reduce hair loss. Theradome® laser hair therapy presents an innovative way to stimulate hair growth, while SMP scalp micropigmentation offers a unique cosmetic procedure to mimic the appearance of hair follicles on the scalp. With these treatment options, individuals in the Toronto GTA region can find effective and personalized solutions to combat hair loss and restore their confidence in their appearance. It is recommended to consult with qualified healthcare professionals and experts in hair loss treatments to determine the most suitable option based on individual needs and preferences.
FIND A TRICHOLOGIST
With the numerous available treatments for hair loss, our Trichologist can recommend the best treatment that can help with your condition. At the Trichology Centre, we have helped patients with all types of hair loss conditions, advising them on the best treatments available and helping them regain their confidence and self-esteem. Early assessment and treatment from the onset of hair loss are important and can help prevent the condition from worsening.
We have two clinics in the Toronto GTA,
TRICHOLOGY CENTRE | hairlossclinic.ca | hairtattoo.ca | laserhairtherapy.ca
600 Sherbourne St #605, Toronto
9140 Leslie St #301, Richmond Hill.
Please give us a call at 647-492-9093
FREE ONLINE HAIR LOSS ASSESSMENT FORM
Are you experiencing any hair loss or scalp issues? Get a Certified Trichologist to assess and help treat your condition in Toronto and Greater Toronto Area. Please complete our online Trichology Assessment Form to get started now.
ADDRESS
Toronto, 225 Wellesley St East #5
Richmond Hill,9140 Leslie St #301
PHONE
(647) 492-9093
hairlossclinic.ca@gmail.com
WORKING HOURS
Mon-Sat 9:00-18:00
Sunday CLOSED
Comments are closed.